|
|
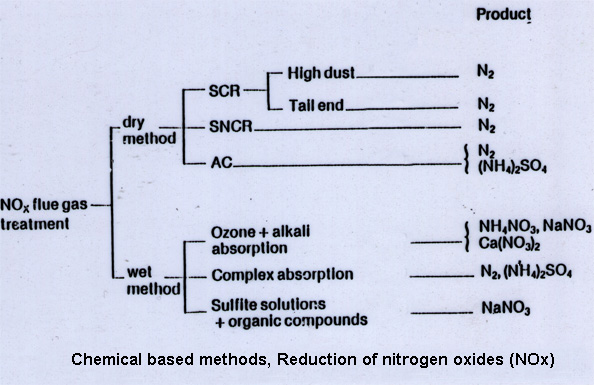
Chemically based NOx-reduction. The ABC&I high technology systems creates a chemical reactor volume inside the NOx-producing boiler or furnace. Examples of used chemical agents and reactions are shown below. The chemical reactions and reactant stoechiometry are controlled by an intelligent software. Advanced construction materials used in the system satisfies requirements in respect to corrosion, temperature, wear and aggressive exposure from chemicals and flue gas. In the many performance tests in past projects NOx-reduction up to 80-90 % has been accomplished, depending on application. Chemical based NOx-reduction techniques can be categorised as either wet or dry. In the wet methods the NOx gas is in direct contact with a chemical solution e.g. in a scrubber. In the dry methods the NOx-reduction is carried out in a gas phase or on a solid surface e.g. in a cathalyst. Examples of wet and dry methods and their end products are given in the scheme below.
The most commonly used NOx-reduction technologies are based on dry methodology. Depending on the used reduction agent a set of chemical reactions are possible. From the given examples below it is evident that system optimisation is an important part of the deNOx implementation. Commonly occurring reactions by NOx-reduction Preferable reaction Less efficient reaction Costly reaction Unwanted reaction Unwanted reaction Commonly occurring reactions by NH3-reduction Preferable reaction Less efficient reaction Costly reaction Unwanted reaction Unwanted reaction |